Core Tip: On March, 2020, National Medical Products Administration (NMPA) announced 5 batches of the information on the registration of Infant Formula (approved), respectively on March 2, 9, 10, 16 and 17. And 2 batches of unapproved infant formula published on March 9 and 17 (18 rejected registration from 3 companies).
Global Foodmate summarised the published information of approved Infant Formula in March, 2020, involving 41 formulation alteration from 9 companies and 21 newly approved formulation from 3 companies.
The situation of approved infant formula in March, 2020 is as follows:
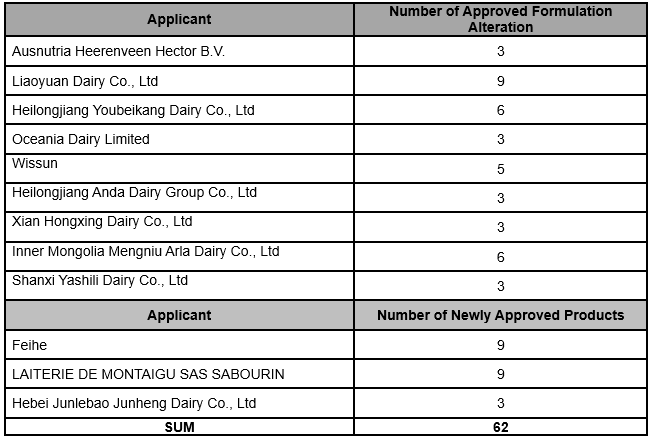
Infant Formula Registration Information (March 2, 2019)
Infant Formula Registration Information (March 9, 2019) (approved + unapproved)
Infant Formula Registration Information (March 10, 2019)
Infant Formula Registration Information (March 16, 2019)
Infant Formula Registration Information (March 17, 2019) (approved + unapproved)
Please note: Original English article of Business Division of Food Safety and Regulatory Compliance of Global Foodmate, please indicate the source from the Global Foodmate if reprint.
Business Division of Food Safety and Regulatory Compliance of Global Foodmate provides food standards & regulations research, labelling compliance consulting/Chinese label design, industry public opinion monitoring and analysis, registration services (of Infant formula, FSMP, Health food, Novel Food Ingredients, Novel Food Additives, New Varieties of Food-Related Products and Overseas manufacturers of imported food) and other comprehensive food safety solutions for domestic and overseas enterprises and institutions in food industry.
Please feel free to contact us: +86 10 68869850, E-mail: global_info@foodmate.net


